Elevating expectations
ABOUT RIVOCERANIB
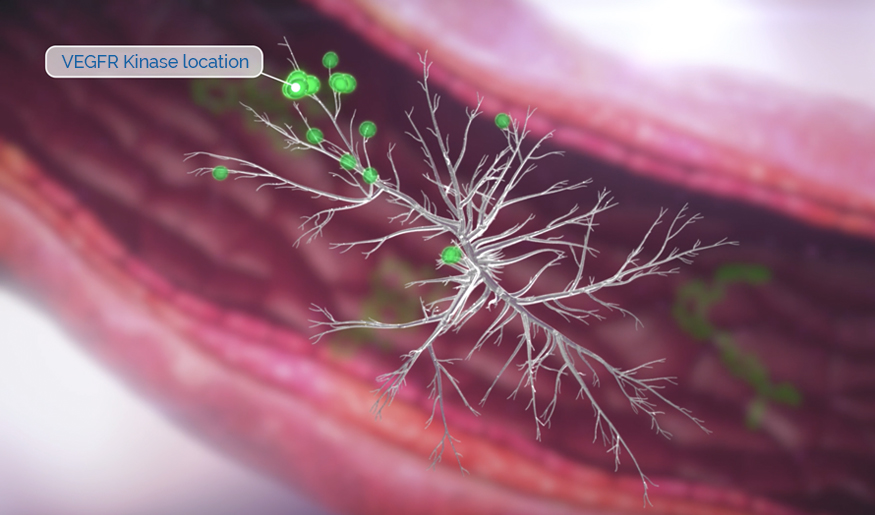
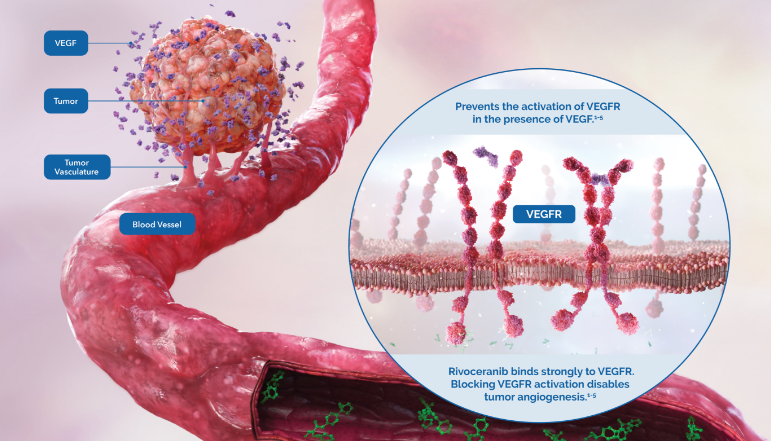
Rivoceranib is a highly potent inhibitor of vascular endothelial growth factor receptor 2 (VEGFR-2), the primary pathway for tumor angiogenesis.1
VEGFR-2 inhibition is a clinically validated approach to limit tumor growth and disease progression.1
Rivoceranib was the first small-molecule tyrosine kinase inhibitor (TKI) approved as a gastric cancer treatment option under the name apatinib in China in December 2014. Apatinib is currently marketed in China for advanced gastric cancer by Jiangsu Hengrui Medicine Co., Ltd., under the brand name Aitan®.2
Today, rivoceranib has been studied in more than 6,000 patients worldwide and was well tolerated in clinical trials with a comparable safety profile to other TKIs.2,3
Elevar Therapeutics holds the global rights (excluding China) and has partnered for the development and marketing of rivoceranib with HLB-LS in South Korea.2
Studied in over 6,000 patients worldwide3
CLINICAL STUDIES
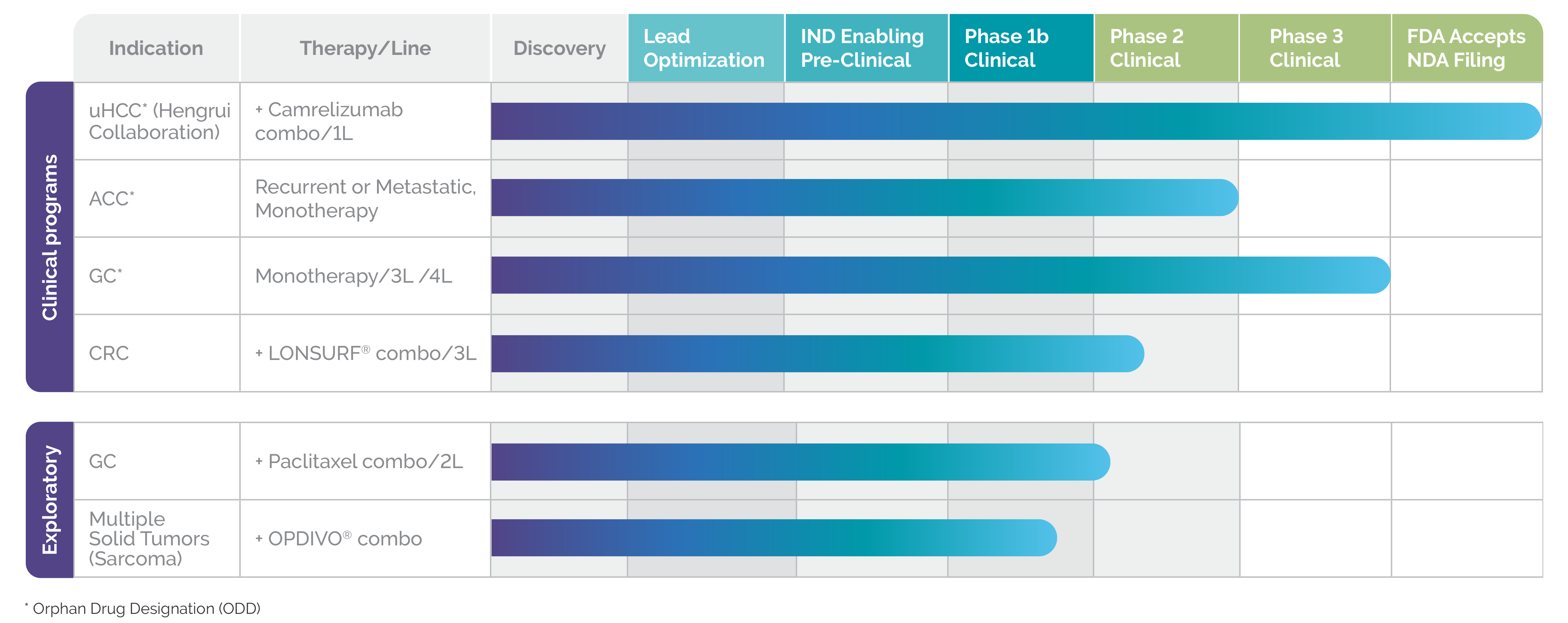
Currently, rivoceranib is being studied both as a monotherapy and in combination with chemotherapy and immunotherapy.2
Clinical studies are planned or underway in multiple tumor types including:
- Hepatocellular carcinoma (in combination with camrelizumab)
- Adenoid cystic carcinoma
- Colorectal cancer (in combination with LONSURF®)
- Gastric cancer
Orphan drug designations for rivoceranib have been granted in gastric cancer (in the US, EU, and South Korea), in adenoid cystic carcinoma (in the US), and hepatocellular carcinoma (in the US).2
MECHANISM OF ACTION (MOA) OF RIVOCERANIB
Studies have shown that rivoceranib is a highly potent inhibitor of VEGF-mediated VEGFR-2 activation.1
Rivoceranib displayed minimal off-target tyrosine kinase inhibitor activity—allowing for a more selective tyrosine kinase receptor inhibition profile.3,4
SEE THE RIVOCERANIB MOA AT A GLANCE
Want to learn more? Contact us
RIVOCERANIB: A PIPELINE IN A PRODUCT
Clinical and exploratory studies in progress
ELEVATE YOUR INTEREST
See the latest news, publications, and events featuring rivoceranib and Elevar Therapeutics.
All product and company names are trademarks™ or registered® trademarks of their respective holders. Use of them does not imply any affiliation with or endorsement by them.
References: 1. Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374-1380. 2. Markets Insider. Elevar Therapeutics Receives Orphan Drug Designation from FDA for Rivoceranib for the Treatment of Hepatocellular Carcinoma (HCC). Published November 11, 2021. Accessed June 1, 2023. https://markets.businessinsider.com/news/stocks/elevar-therapeutics-receives-orphan-drug-designation-from-fda-for-rivoceranib-for-the-treatment-of-hepatocellular-carcinoma-hcc-1030965744 3. Data on file. Elevar Therapeutics; 2021. 4. Data on file. Elevar Therapeutics; 2022.