Elevating expectations
ABOUT CAMRELIZUMAB
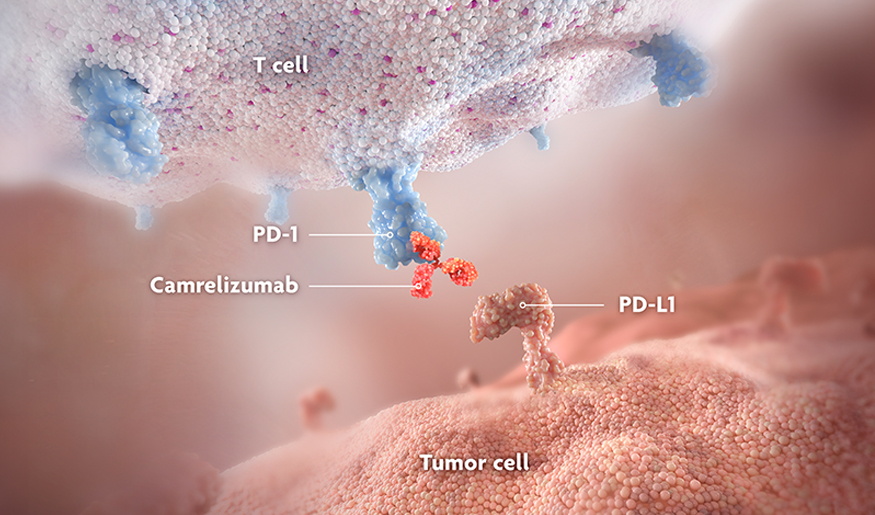
Camrelizumab is a PD-1-blocking antibody, which prevents the binding of PD-1 on T and B lymphocytes (T and B cells) and natural killer cells (NK cells) to programmed cell death ligand 1 (PD-L1) expressed on tumor cells.1
The US Food and Drug Administration (FDA) granted Orphan Drug Designation to camrelizumab for hepatocellular carcinoma (HCC) in April 2021.2
Elevar holds the global rights to commercialize camrelizumab in combination with rivoceranib for unresectable hepatocellular carcinoma (uHCC), with the exception of China and South Korea.3 Read more about this global commercial licensing agreement here.
Studied in over 5,000 patients worldwide4,5
MECHANISM OF ACTION (MOA) OF CAMRELIZUMAB
Camrelizumab blocks the PD-1 pathway in the tumor microenvironment, which activates and reinvigorates T cells. This allows the immune system to mount an anti-tumor response.1,6,7
SEE THE CAMRELIZUMAB MOA AT A GLANCE
Want to learn more? Contact us
ELEVATE YOUR INTEREST
See the latest news, publications, and events featuring camrelizumab and Elevar Therapeutics.
References: 1. Markham A, Keam SJ. Drugs. 2019;79(12):1355-1361. 2. Orphan drug destinations and approvals for camrelizumab. US Food and Drug Administration. Accessed November 9, 2023. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=675718 3. Elevar Therapeutics and Jiangsu Hengrui Pharma announce global commercialization licensing agreement for PD-1 inhibitor camrelizumab in combination with rivoceranib for uHCC. Elevar Therapeutics. Accessed November 9, 2023. https://elevartx.com.com/2023/10/17/elevar-therapeutics-and-jiangsu-hengrui-pharma-announce-global-commercialization-licensing-agreement-for-pd-1-inhibitor-camrelizumab-in-combination-with-rivoceranib-for-uhcc/ 4. Elevar Therapeutics. Press release. Accessed November 30, 2023. https://elevartx.com.com/2023/07/17/elevar-therapeutics-announces-fda-acceptance-for-filing-of-new-drug-application-for-rivoceranib-in-combination-with-camrelizumab-as-a-first-line-treatment-for-unresectable-hepatocellular-carcinoma/ 5. Qin S, Chan SL, Gu S, et al. Lancet. 2023;402(10408):1133-1146. doi:10.1016/S0140-6736(23)00961-3 6. Buchbinder EI, Desai A. Am J Clin Oncol. 2016;39(1):98-106. 7. Jiang Y, Chen M, Nie H, Yuan Y. Hum Vaccin Immunother. 2019;15(5):1111-1122.