View our Abstracts from the 2024 ASCO Annual Meeting
In-Person Poster Presentation
June 1, 2024
1:30 PM to 4:30 PM CDT
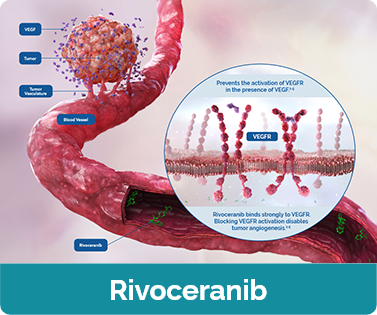
View the Complementary MOAs of Camrelizumab + Rivoceranib
WHAT ELSE IS HAPPENING IN THE BOOTH?
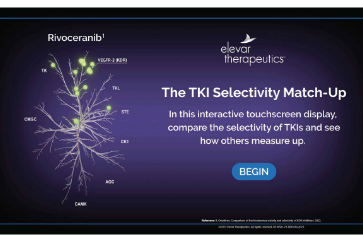
Experience the “TKI Selectivity Match-up”
In this interactive touchscreen display, you can compare the selectivity of TKIs and see how others measure up to rivoceranib.

Review the latest uHCC data
Talk to a team member in the booth about what’s new in uHCC treatment.
Stay connected
Sign up to receive important information about camrelizumab + rivoceranib, company news, and updates or to connect with a member of our team.
*Required field
References: 1. Qin S, Chan SL, Gu S, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402(10408):1133-1146. 2. Jang S, Strickland B, Finis L, et al. Comparative biochemical kinase activity analysis identifies rivoceranib as a highly selective VEGFR2 inhibitor. Cancer Chemother Pharmacol. 2023;91(6):491-499.